Therefore, realization of hydrogel-forming polymer compositions having relatively low levels of extractable polymer material is an important feature of the present invention. In its article-of-manufacture aspects, the present invention relates to an absorbent structure suitable for use in disposable absorbent articles. USB2 en. The hydrogel formed from this powder had a shear modulus of 1. Olefinically unsaturated sulfonic acid monomers include aliphatic or aromatic vinyl sulfonic acids such as vinylsulfonic acid, allyl sulfonic acid, vinyltoluenesulfonic acid and styrene sulfonic acid; acrylic and methacrylic sulfonic acid such as sulfoethyl acrylate, sulfoethyl methacrylate, sulfopropyl acrylate, sulfopropyl methacrylate, 2-hydroxyacryloxy propyl sulfonic acid, 2-hydroxymethacryloxy propyl sulfonic acid and 2-acrylamidomethyl propane sulfonic acid. Buying options Chapter EUR Generally the aqueous reaction mixture will contain no more than about 50 mole percent based on total monomer present of these optional non-acid comonomers, and preferably no more than about 25 mole percent. The absorbent composite according to claim 1, wherein the superabsorbent material is in the form of discrete particles. The median pore size of nonwoven fibrous webs, including fluffed pulp batts as taught herein, can be determined for example by using the method taught by Burgeni, et al. Five hundred forty parts of doubly distilled water, 60 parts of acrylic acid, and 1. The amount of polymerized acid moieties e. While maintaining external cooling at 6° C.


Absorbent composites were made according to Example I, using hydrocolloid particles obtained as IMP, lot no. AUA1 en. Of all of these types of cross-linking agents, the most preferred for use herein are the di- or poly-esters of unsaturated mono- or polycarboxylic acids with polyols, the bisacrylamides and the di- or triallyl amines. The term "density" as used herein refers to the density of the composite absorbent structure 16 and not just the fiber density. This percentage of the total monomers utilized which are neutralized acid group-containing monomers is referred to herein as the "degree of neutralization. Cross-linking agents of many of the foregoing types are described in greater detail in the hereinbefore-referenced U. J Appl Polym Sci 2 — More preferably, gel volume, v, and shear modulus, s, of hydrogels formed from this "especially preferred" class of polymers will be defined by the equation:. Cross-linking agents having at least two polymerizable double bonds include i di- or polyvinyl compounds such as divinylbenzene and divinyltoluene; ii di- or poly-esters of unsaturated mono- or poly-carboxylic acids with polyols including, for example, di- or triacrylic acid esters of polyols such as ethylene glycol, trimethylol propane, glycerine, or polyoxyethylene glycols; iii bisacrylamides such as N,N-methylenebisacrylamide; iv carbamyl esters that can be obtained by reacting polyisocyanates with hydroxyl group-containing monomers; v di- or poly-allyl ethers of polyols; vi di- or poly-allyl esters of polycarboxylic acids such as diallyl phthalate, diallyl adipate, and the like; vii esters of unsaturated mono- or poly-carboxylic acids with mono-allyl esters of polyols such as acrylic acid ester of polyethylene glycol monoallyl ether; and viii di- or triallyl amine. Positioned on the top of the backing sheet is an hourglass-shaped absorbent core comprising the absorbent structure of the present invention.
Buying options
Aliquots of the hydrogel-forming polymer to be tested are swelled in i 20 parts of Synthetic Urine SU solution and ii 20 parts of Blue Dextrin BD solution. Cross-linking agents of many of the foregoing types are described in greater detail in the hereinbefore-referenced U. After 7 minutes, the viscosity of the solution prevented further stirring. Such a situation, of course, then contributes to undesirable leakage of body fluid from the article. The following equation is used to calculate the gel volume: EQU1. In a cooled flask, It is estimated that these surges correspond to the average bladder capacity of the infants being tested. In a slow, dropwise manner, a portion of the contents of the addition funnel was added until a 0. Waste solution solidifying agent, process for preparing the same and use of the same. Inert gas was introduced to expel dissolved oxygen therefrom, and the temperature was raised to 40° C. Such hydrogel-forming polymer compositions are substantially water-insoluble, slightly cross-linked, partially neutralized polymers which are prepared from unsaturated polymerizable, acid group-containing monomers and cross-linking agents. Reprints and permissions.
Cellulose-Based Hydrogel for Personal Hygiene Applications | SpringerLink
- Typically the basis weight of the absorbent structures herein can range from about 0.
- Under these conditions, an oscillatory torque stress is applied via the upper conical element to the swollen hydrogel.
- Of all the foregoing unsaturated, acid-containing monomers, preferred monomers include acrylic acid, methacrylic acid, and 2-acrylamidomethyl propane sulfonic acid.
- Two or more different monomer types of the hereinbefore described acid group-containing monomers may be copolymerized in order to provide hydrogel-forming polymer material of this requisite acid group-containing monomer content.
- LTB en.
- Powdery, cross-linked absorbent polymers method for the production thereof and their use.
Year of fee payment : 4. Year of fee payment : 8. Year of fee payment : Effective date : The present invention relates to improved hydrogel-forming polymer compositions which can be used as absorbents in absorbent structures and absorbent articles such as diapers, sanitary napkins and the like. Such hydrogel-forming polymer compositions are substantially water-insoluble, slightly cross-linked, partially neutralized polymers which are prepared from unsaturated polymerizable, acid group-containing monomers and cross-linking agents. These hydrogel-forming polymer materials, upon imbibing fluids, form hydrogels. Such polymer materials have relatively high gel volume and relatively high gel strength as measured by shear modulus of the hydrogen which forms therefrom. Such polymer materials also contain relatively low levels of extractable polymer material which can be extracted therefrom by contact with synthetic urine. Preferred hydrogel-forming polymers having these characteristics can be prepared by polymerizing the acid group-containing monomers in their free acid form at relatively low monomer concentrations, preferably using relatively low polymerization temperatures. Absorbent structures and absorbent articles containing these dried hydrogel-forming polymer materials are also disclosed. This application is a reissue of Ser. This invention relates to improved hydrogel-forming polymer compositions and to a process for their preparation. Such hydrogel-forming polymers are those which, upon contacting fluids i. These hydrogel-forming polymer materials are useful as absorbents in absorbent structures which can be incorporated into absorbent articles such as disposable diapers, adult incontinence pads, sanitary napkins and the like.
Personal hygiene product is an inseparable part of urban society. It has given comfort, reliability, and flexibility to sick people, women, and children. The hygiene items containing superabsorbent polymer hydrogels for absorbing large amount of body fluids are the attractive inventions of modern science. The hydrogels swell and imbibe body fluids in the presence of hydrophilic functional groups in the polymeric backbone, pampers and tampons hydrogels. Current trend of using acrylate-based superabsorbent in hygiene products is creating significant portion of urban garbage. This pile up is not pampers and tampons hydrogels shrinking land sites but also harming a lot to the environment due to non-degradability of superabsorbent materials existing in the core of hygiene product. In spite of high water-holding capacity of petrochemical-based superabsorbent polymer, it has a hidden curse on nature of non-degradability and health risk. Pampers and tampons hydrogels is the most abundant biocompatible matter on this earth which basically originated from plants. It is also naturally occurring long chain polymer that plays a vital role in food cycle in animal kingdom.
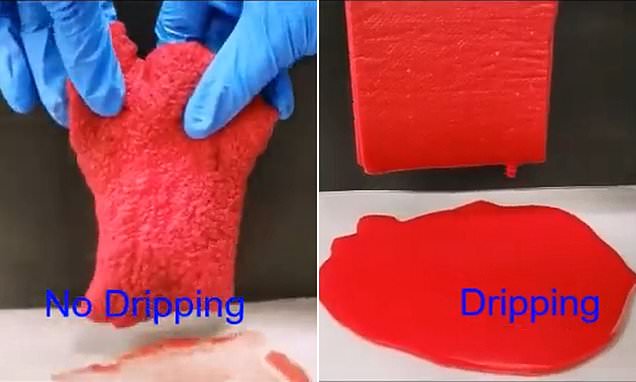

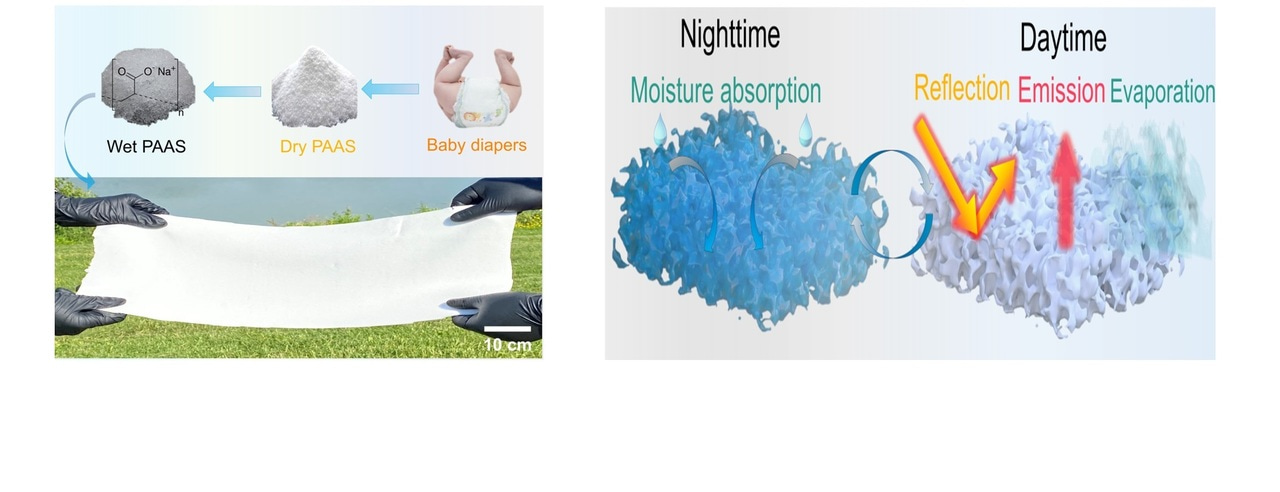
Pampers and tampons hydrogels. Hydrogels: a journey from diapers to gene delivery
Kind code pampers and tampons hydrogels ref document : A1. Effective date : Kind code of ref document : B1. Ref document number : Country of ref document : DE. Date of ref document : Ref country code : GB. Ref legal event code : E. Ref country code : ES. Ref legal event code : FG2A.
Publication types
.
The article may be provided with adhesive tape fasteners not shown of the type known in the art to secure it about the body.


MAKING FERTILIZERS OUT OF DIAPERS!? (HYDROGEL)
In my opinion you are not right. Let's discuss.
Very amusing opinion